Biodiesel PRODUCTION
Biodiesel is a biofuel that is similar to diesel and is derived from natural lipids. The chemical industry obtains biodiesel through esterification of vegetable oils or animal fats with monovalent alcohols such as methanol or ethanol.
THE ProcesS
Three ingredients are needed for the production.
1. Oil: Vegetable oils such as sunflower/soybean oil or animal fat as it occurs as waste in restaurants or coffee shops.
2. Alcohol: Methanol is generally used for processing recycled oils. With new oil the mixture with ethanol is possible.
3. Catalyst: KOH or NaOH can be used. Both have advantages. The remaining glycerin from the KOH process is less toxic and dissolves much better in methanol. On the other hand, NaOH is simple and inexpensive to get.
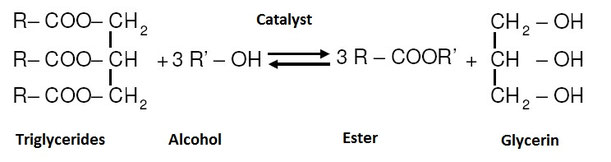
Transesterification of triglycerides is what the process for the production of biodiesel is called. Low molecular weight alcohols react with triglyceride molecules to esters and glycerin.
The biodiesel equipment is composed of two reactors, the main one for the transesterification with a capacity of 70 gallons. The second one for the methoxide mixture. It also has filtration systems with ionized particles to remove traces of soap. ISA University recycles 50 gallons of cooking oil per month. For its later conversion to biodiesel being used in the fleet of vehicles of the institution.
For research purposes we use a 'Biodiesel Starter Kit' with a capacity of 7 ounces. It allows a high flexibility due to a lower amount of used raw material and experiments can be repeated more rapidly to optain exact data.because so much raw material is not needed and you can make repetitions very fast to obtain more exact data.
The tests carried out on biodiesel include: titration test for oil acidity, density, conversion of fatty acids, fog point, traces of soap.




